RMS Foundation
Robert Mathys-Strasse 1
2544 Bettlach
Switzerland
Phone +41 32 644 2000
Our focus is on the satisfaction and success of our customers, research partners and employees. We wish that everyone who deals with us can say: "RMS brings us forward. They are the best."
We want to achieve this through
Expertise and competence: a high level of expertise, continuous education and motivation are the basis for high-quality service and research.
Impartiality and integrity: We always act impartially, fairly and transparently to ensure that our tests and analyses are free from any influence.
Continuous improvement: We strive for continuous improvement by regularly reviewing our processes, obtaining customer feedback and implementing optimization measures. For us, recognized errors are opportunities for improvement.
Precision and accuracy: We use modern methods and technologies to deliver consistent and reliable results that meet the highest quality standards.
Partnership: We respond to our customers' wishes and are a reliable partner in finding solutions to current tasks and future challenges.
Research and knowledge transfer: Through our research, we are committed to contributing to the advancement of medical devices, manufacturing processes and analytical technologies.
We have been an ISO/IEC 17025 accredited testing laboratory since 1995. In August 2013, we received type C accreditation. This allows us to develop special, non-standardized test methods and offer tailor-made solutions without the need for prior assessment by the accreditation body. We employ qualified technologies and validated methods in this process.
Accreditation is the official recognition of our competence to perform services reliably and correctly, which creates trust and transparency. This short film from ILAC/IAF highlights the benefits of accreditation.
Our accreditation is renewed every five years through a re-accreditation audit. Two surveillance audits take place in between. The assessments are carried out by the Swiss Accreditation Service SAS.
Our management system has been ISO 9001 certified since 1993. This certification ensures that we consistently deliver high-quality services that meet customer requirements as well as legal and regulatory requirements.
The certificate is renewed every three years through a re-certification audit, with annual surveillance audits conducted in between. These audits are carried out by an independent certification body, in our case QS Zurich.
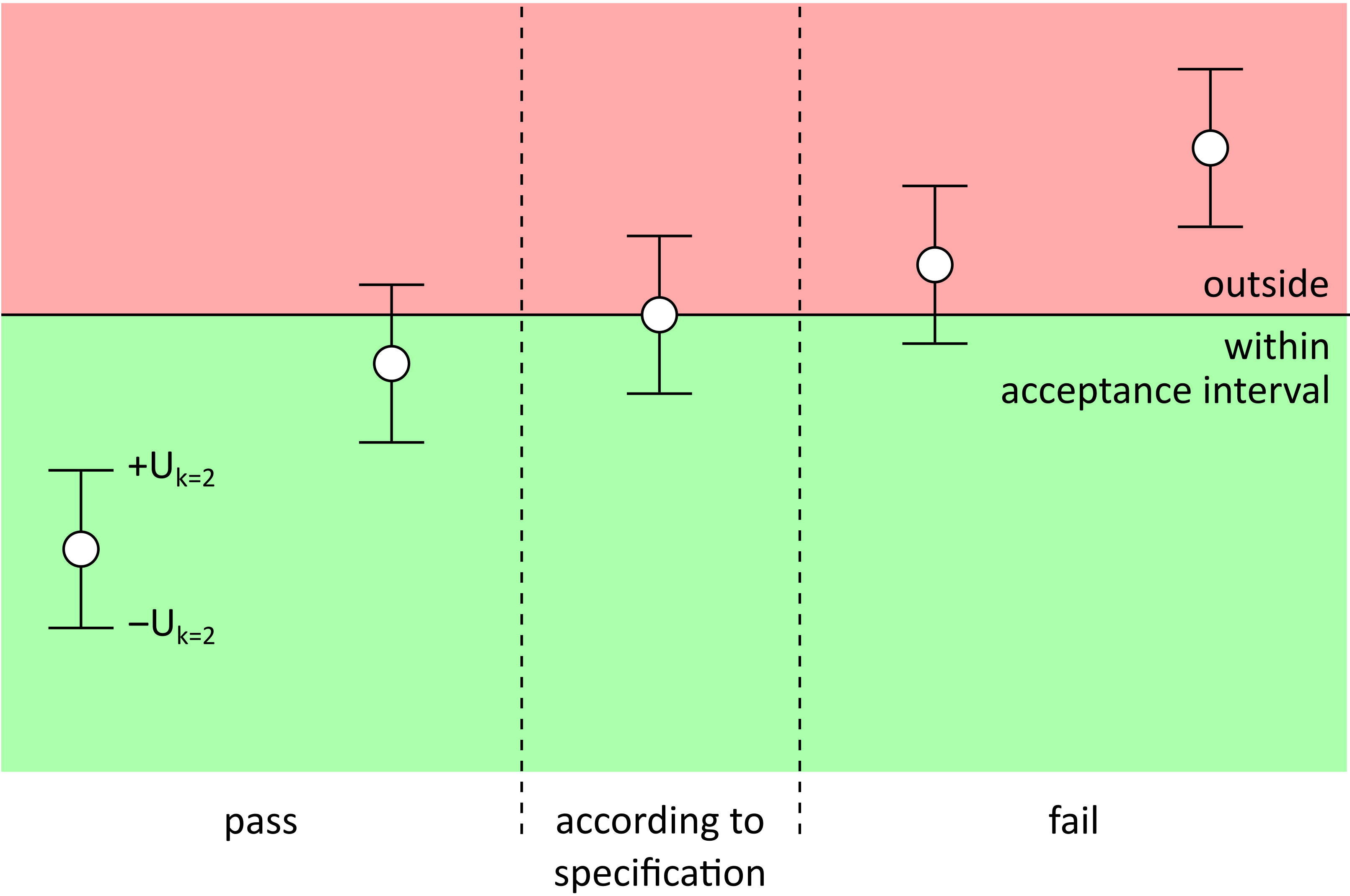
Every measurement result is subject to a degree of measurement uncertainty, as the measured value is always an estimate of the true (unknown) value due to technical limitations and environmental influences.
Unless otherwise specified or agreed upon, we make statements about compliance with a given reference or with a limit value (conformity assessment) without taking measurement uncertainty into account.
Since 1995, the services of our materials testing laboratory have been accredited according to ISO/IEC 17025. Our QM system is ISO 9001 certified.
Here you will find our latest blog posts.

RMS Foundation
Robert Mathys-Strasse 1
2544 Bettlach
Switzerland
Phone +41 32 644 2000
E-Mail
The RMS Foundation will be closed from 24 December 2025 through 2 January 2026. We will be pleased to assist you again from Monday, 5 January 2026.
Subscribe to our Info-letter, and we will inform you about 10 times a year about current developments in the fields of material testing, research, and knowledge transfer.